Methanol from Biomass Fact Sheet
Introduction and basic data on methanol
Production process for methanol
State of the Art for methanol production
Major stakeholders in methanol in the EU
Introduction
Methanol, also known as methyl alcohol, wood alcohol, or wood spirits, is often abbreviated as MeOH. It is the simplest alcohol, and is a light, volatile, colourless, flammable liquid with a distinctive odour. At room temperature it is a polar liquid. MeOH is miscible with water, petrol and many organic compounds. MeOH burns with an almost invisible flame and is biodegradable. Without proper conditions, methanol attracts water while stored. Methanol is a safe fuel. The toxicity (mortality) is comparable to or better than gasoline. It also biodegrades quickly (compared to petroleum fuels) if spilled.
Molecular Formula![]()
Comparison of Fuel Properties
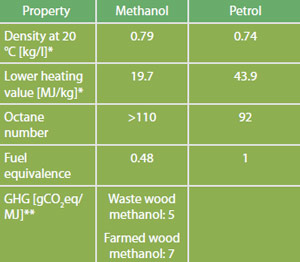
Source: *FNR 2012. Median values are used for simplification. Please refer to standards for ranges. **Directive 2009/28/EC, total for cultivation, processing, transport and distribution.
Utilization
Chemical feedstock, petrol blend component
Relevant fuel regulations
EN 228
Main feedstocks
Natural gas, coal, biomass
Scale of Production
Industrial scale
Production process
In nature MeOH is produced via anaerobic metabolism by many bacteria. It is also formed as a by-product during the ethanol fermentation process. MeOH also occurs naturally in many plants, especially in fruits. MeOH is mainly synthesized from natural gas, but also from coal, mainly in China and South Africa. Biomass can be converted to MeOH via thermochemical and biotechnological pathways as shown in the following diagram.
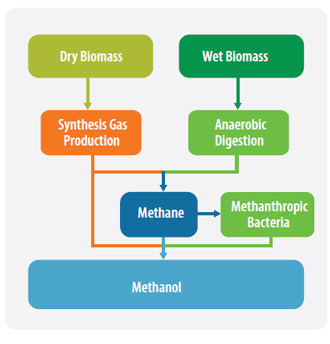
Thermochemical pathways
The thermochemical conversion paths to MeOH are basically the same as for fossil feedstocks, such as coal or natural gas.
The biomass is gasified and the resulting synthesis gas, a mixture of CO, H2 and CO2 is adapted to the quality requirements of MeOH synthesis.
During synthesis the following reactions occur:
CO + 2H2![]() CH3OH
CH3OH
CO2 + 3H2![]() CH3OH + H20
CH3OH + H20
CO2 + H2![]() CO + H20
CO + H20
The formation of MeOH is exothermic and is favoured by high pressures and low temperatures. For reasons of process simplification, investment cost reduction and energy consumption reduction, alternatives are under development, which could also be used for MeOH from biomass:
Direct oxidation of Methane: 2CH4 + O2![]() 2CH3OH
2CH3OH
Liquid-phase oxidation of Methane
Conversion through monohalogenated methanes
Biochemical pathways
One biochemical route is via methane formation by anaerobic digestion. This process is well developed due to the rise of biogas production from municipal waste or landfill sites.
The biogas has to be cleaned to obtain a gas with high methane content and MeOH is then produced from the methane as described above.
Recently a genuine biochemical route using methanothrophic bacteria has been investigated. For example, bacteria such as Methylococcus capsulatus will convert methane to MeOH if methane is the only available resource.
State of the Art
MeOH has grown into one of the largest chemical synthesis feedstocks. Key uses include production of formaldehyde, MTBE/TAME (petrol additives), acetic acid, DME and olefins and direct use as a petrol blend component.
In 2007 the world production of MeOH amounted to 40 million tonnes with a forecast compound annual growth rate of 4.2 % for the period 2008-2013 excluding captive production for the methanol-to-olefins (MTO) route. Today, methanol from biomass is produced through gasification of glycerine, a by-product of biodiesel production, by BioMCN in the Netherlands. The thermochemical conversion of syngas to methanol is well known from fossil feedstocks and the basic steps are not different for biomass. The main issue faced is the economic feasibility of gasification of biomass at elevated pressures and conditioning of the raw synthesis gas.
In the past there was some small-scale production of methanol from biomass. In 2004 the German company Choren Industries GmbH produced methanol from wood using its Carbo-V process. In the Chemrec AB pilot plant in Piteå, Sweden about 6 tons per day of methanol is used as an intermediate in the production of BioDME. While the biochemical route through methanothrophic bacteria is still in an early state of development the conversion of biogas to methanol has been proven at bench scale. ZSW has proven that methanol could be produced from biogas at a decentralised level.
Applications of methanol
Low-percentage methanol-gasoline blends (up to 3 % as per current EU standard EN228) can be effectively used in conventional spark-ignition engines with no technical changes, the use of alcohol fuels in heavy duty applications is being investigated by motor manufacturers.
Projects on methanol
See R&D Funding page for further project details
SUPER METHANOL - Reforming of crude glycerine in supercritical water to produce methanol for re-use in biodiesel plants (FP7-212180)
MTO/OCP Project - Methanol-to-olefins/olefin cracking process (€45m project funded by Total)
Major stakeholders
Some major methanol stakeholders in the EU are listed below:
Chemrec AB, Sweden
VärmlandsMethanol AB, Sweden
BioMCN B.V., Netherlands
B.T.G. BIOMASS TECHNOLOGY GROUP BV, Netherlands
Choren Industries GmbH, Germany
Karlruhe Institute for Technology (KIT), Germany
ZSW, Germany
DECHEMA, Germany
Technical University of Vienna, Austria
Technical University of Graz, Austria
Further Information
See the Methanol page for updated project information
